Scientific calendar January
Severe COVID-19 infection
Which are known factors triggering the appearance of NRBC in the peripheral blood of adults?
Iron deficiency anaemia
Increased erythropoietin levels
High levels of pro-inflammatory cytokines (e.g. IL-6)
Arterial hypoxaemia
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background
Critically ill patients with respiratory infections can develop acute respiratory distress syndrome (ARDS), a severe complication and potentially life-threatening condition. Hence, reliable prognostic tools to predict respective outcomes early on are highly desired by clinicians [1].
Nucleated red blood cells (NRBC) are the progenitor cells of the red blood cells, which are absent in the peripheral blood of healthy adults. Under haemopoietic stress, however, NRBC can be found in the peripheral blood, since the bone marrow also releases the red blood cell lineage in the premature stages. When NRBC are present in blood of critically ill patients, this is generally associated with increased mortality [2]. Interestingly, NRBC positive patients showed significantly lower levels of arterial oxygen partial tension during intensive care treatment than NRBC negative patients [3]. Lower arterial oxygen partial tension has been found to indicate the future presence of NRBC. Moreover, findings in critically ill patients suffering from ARDS suggest that NRBC are equally predictive in ARDS mortality [4]. Additionally, in patients with acute pancreatitis, immature granulocytes (IG) could facilitate the identification of patients with a high risk of developing ARDS [5]. IG was judged as a potential indicator of ARDS incidence and has shown predictive power similar with (or greater than) other biomarkers.
The recent SARS-CoV-2 pandemic put a strain on hospitals and especially intensive care units which had to cope quickly with the rising numbers of hospitalised COVID-19 patients.
To emphasise this bottleneck and support hospitals with the management of COVID-19 patients, the COVID-19 prognostic score was developed [1]. The score is based on 10 variables, available directly or indirectly from the XN-Series analysers, which are weighted with points according to the measured result
(see Table 1).
Score values generated within the first three days of hospital admission can predict clinical severity in COVID-19 patients over the following two weeks. The score performance was shown to be superior to single parameters or parameter ratios.
Table 1 Overview of haematological variables utilised for the COVID-19 prognostic score.
| Variables | |
| Primary variables | Immature granulocytes-to-lymphocytes ratio: IG/L*100 Neutrophils-to-lymphocytes ratio: N/L Reactive monocytes as percentage of monocytes: RE-MONO/M Antibody-synthesizing lymphocytes as percentage of lymphocytes: AS-LYMPH/L Haemoglobin equivalent difference between the reticulocytes and mature red blood cells: Delta-He Nucleated red blood cells: NRBC |
| Secondary variables | Haemoglobin: HGB Percentage of hypochromic cells: Hypo-He Platelet count: PLT Immature platelet fraction count: IPF |
Numerical results
A 56-year-old male patient was hospitalised due to strong symptoms associated with his COVID-19 infection. The development of the haematological parameters measured on an XN-Series analyser was observed over a period of 33 days.
Upon admission (day 0), the CBC results revealed almost normal results, but only the red blood cells, haemoglobin and haematocrit were slightly above the reference intervals. The automated white blood cell differential showed a normal distribution apart from few lymphocytes with increased fluorescence signals visible in the upper part of the WDF scattergram.
Already on day 2, the leucocytosis was noted (WBC 14.65 x 10³/µL) with neutrophilia. However, the neutrophils did not show signs of activation (NEUT-RI 42.6 FI, NEUT-GI 153.6 SI within the reference intervals [6]).
On day 4, the WBC count reached 17.89 x 10³/µL, and the analyser indicated the presence of IG with the ‘IG Present’ flag because the percentage of these cells increased to 5.1%.
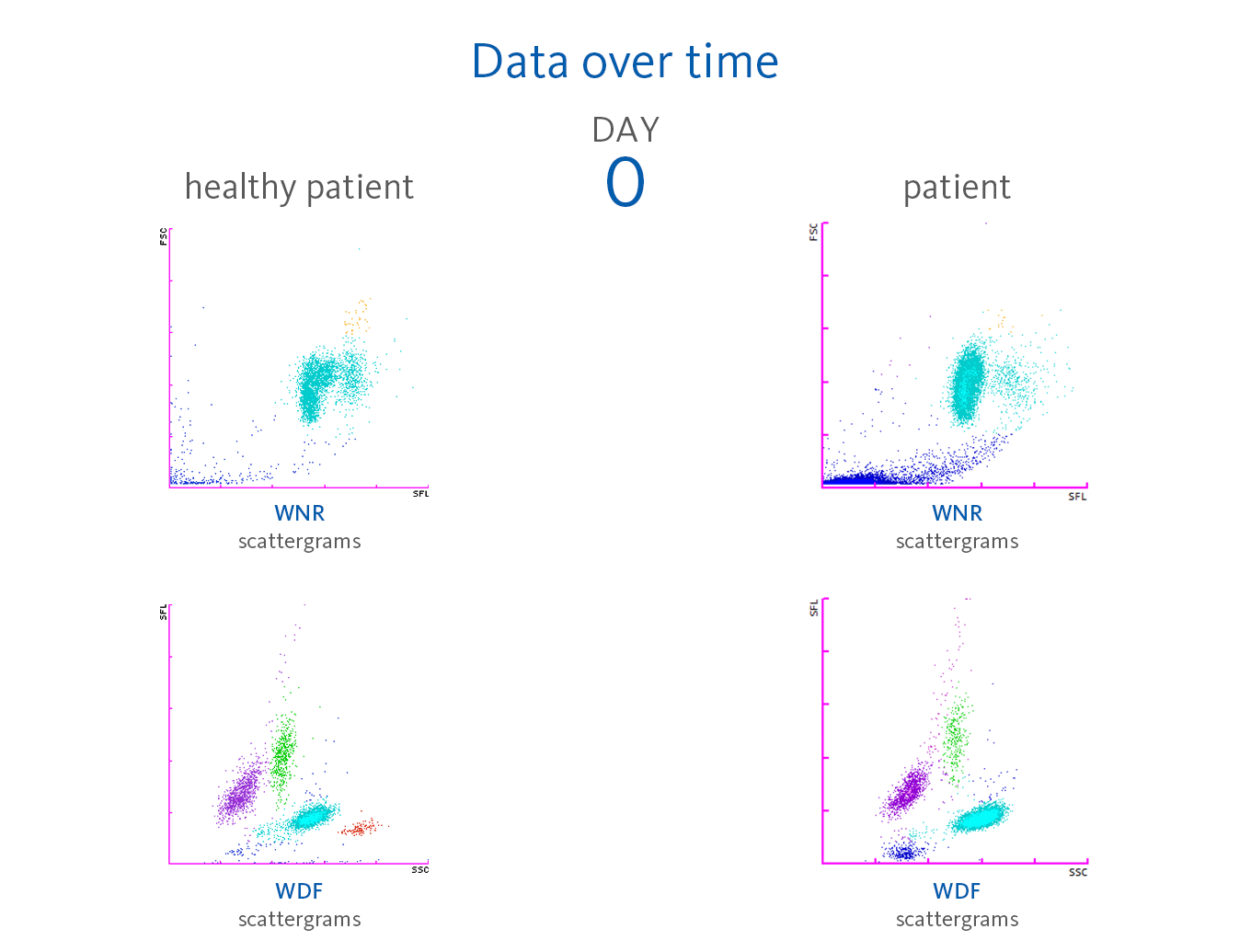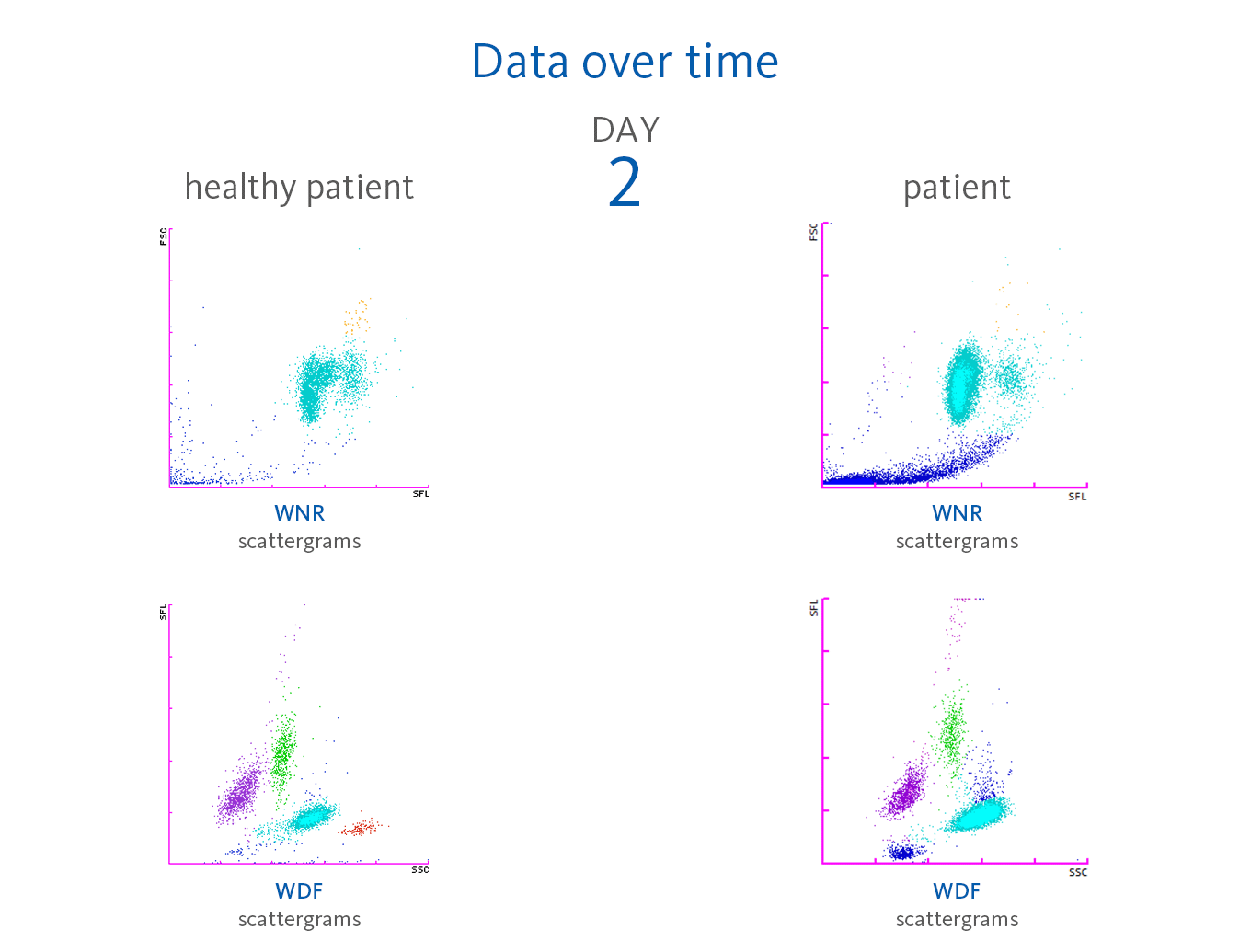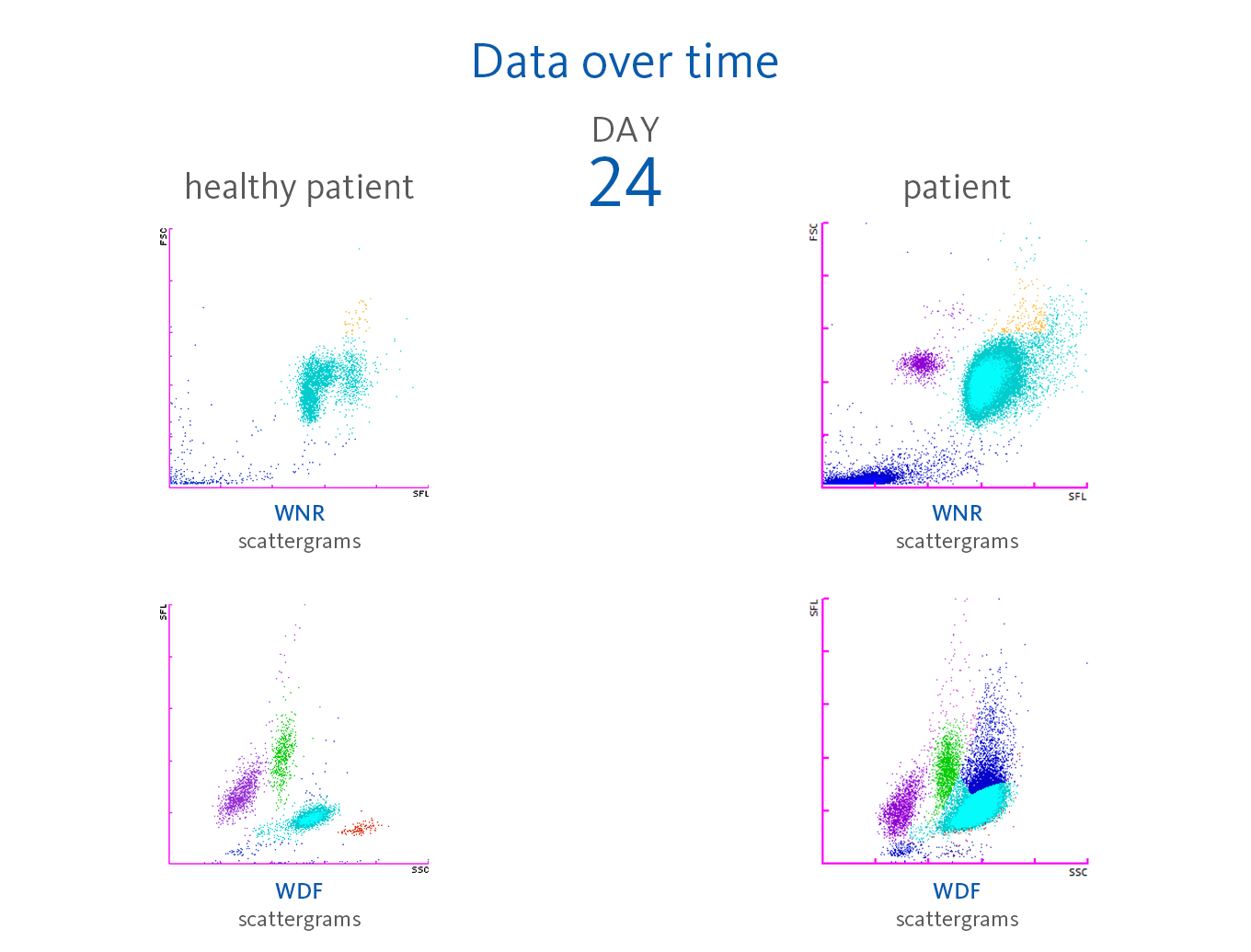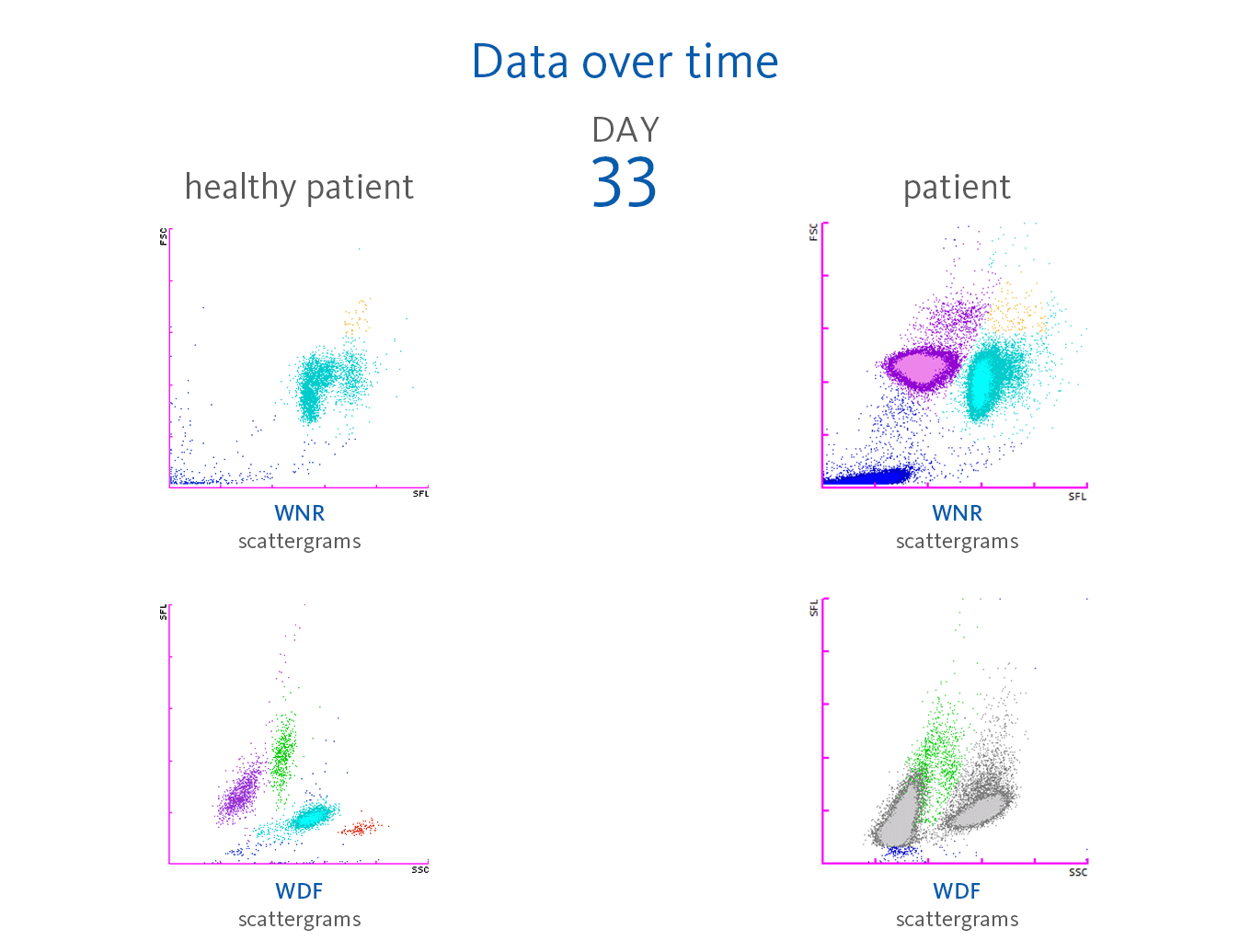
Leucocytosis developed to even higher numbers over the course of time, reaching levels of > 20.00 x 10³/µL on day 11 and > 30.00 x 10³/µL on day 17 with IG present. During these three weeks, the red blood cells, haemoglobin and haematocrit continuously decreased, whereas platelets remained within the reference range, although showing already a downward trend. On day 24, the first time a significant presence of NRBC was recorded with 280 cells/µL (0.7%) as well as an increase in neutrophil activation (NEUT-RI). The presence of around 2% NRBC was recorded for six consecutive days until a remarkable increase happened:
- day 31: 11% NRBC
- day 32: 47.2% NRBC
- day 33: 116.5% NRBC
On day 26, the platelets dropped the first time to below 100 x 10³/µL, reaching the nadir on day 33 with 31 x 10³/µL. A meta-analysis by Lippi et al. found that platelet count may be a simple, economic, rapid and commonly available laboratory parameter that could straightforwardly discriminate the severity in COVID-19 patients. Moreover, it was observed that thrombocytopenia is also associated with threefold enhanced risk of severe COVID-19 [7].
Scattergram interpretation
The XN-Series analysers do not only provide absolute and percentage of cell count, but also the parameters that indicate cell activation. The reason for this is the measurement of the side fluorescence light intensity (SFL) of WBC in the WDF channel, indicating increased RNA activity in the cytoplasm of WBC. Sysmex analysers provide a set of parameters that indicate cell activation:
- Neutrophil activation: neutrophil reactivity intensity (NEUT-RI) and neutrophil granularity intensity (NEUT-GI)
- Lymphocyte activation: reactive lymphocytes (RE-LYMP) and antibody-synthesizing lymphocytes (AS-LYMP)
- Monocyte activation: reactive monocytes (RE-MONO)*
Neutrophilia was present from day 2. However, neutrophil activation
(NEUT-RI) was observed only on day 24. Already on admission, in the WDF scattergram of this patient, lymphocytes with higher fluorescence can be recognised. It is known from publications that despite a decrease in lymphocyte count, an increase in certain subpopulations of lymphocytes can be observed in COVID-19 patients. For example, Martens et al. describes that RE-LYMP, AS-LYMP and high fluorescence lymphocyte cells (HFLC) were higher in COVID-19 patients as compared with the controls [8].
Additionally, on admission of the patient, the monocyte population (green) shows an increased fluorescence signal visible by an upward trend of the population in the WDF scattergram (see vertical blue line for comparison). It can be hypothesised that this reflects the activated state of monocytes [8].
Some case reports describe leucoerythroblastic reactions, defined as immature erythroid and immature myeloid cells circulating in the peripheral blood of patients with COVID-19 infections [9,10]. In the blood sample of the presented patient, NRBC was present on day 24 after hospitalisation in the WNR scattergram followed by a drastic increase on day 31, 32 and 33 of the observation period. Also, immature granulocytes (myelocytes and promyelocytes) were present in large numbers.
*The parameter RE-MONO is a service parameter that became available with XN IPU software version 22.16 and can be transmitted to the Extended IPU or LIS.
References
[1] Linssen J et al. (2020): A novel haemocytometric COVID-19 prognostic score developed and validated in an observational multicentre European hospital-based study. Elife. 9:e63195.
[2] Stachon A et al. (2006): Poor prognosis indicated by nucleated red blood cells in peripheral blood is not associated with organ failure of the liver or kidney. Clin Chem Lab Med. 44(8):955–961.
[3] Kuert S et al. (2011): Association of nucleated red blood cells in blood and arterial oxygen partial tension. Clin Chem Lab Med. 49(2):257–263.
[4] Menk M et al. (2018): Nucleated red blood cells as predictors of mortality in patients with acute respiratory distress syndrome (ARDS): an observational study. Ann Intensive Care. 8(1):42–53.
[5] Huang Y et al. (2019): Immature granulocytes: A novel biomarker of acute respiratory distress syndrome in patients with acute pancreatitis. J Crit Care. 50:303–308.
[6] Cornet E et al. (2015): Contribution of the new XN-1000 parameters NEUT-RI and NEUT- WY for managing patients with immature granulocytes. Int J Lab Hematol. 37(5):e123–126.
[7] Lippi G et al. (2020): Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 506:145–148.
[8] Martens R et al. (2021): Hemocytometric characteristics of COVID-19 patients with and without Cytokine Storm Syndrome on the Sysmex XN-10 hematology analyzer. Clin Chem Lab Med. 59(4):783–793.
[9] Mitra A et al. (2020): Leukoerythroblastic reaction in a patient with COVID‐19 infection.
Am J Hematol. 95(8):999–1000.
[10] Milanesio M et al. (2021): Leukoerythroblastic reaction associated with COVID-19 infection. Case report. Rev Fac Cien Med Univ Nac Cordoba. 78(1):64–67. Article in Spanish.
Case report

This clinical case reports the disease progression of a patient hospitalised due to a SARS-CoV-2 infection. The patient’s condition worsens significantly over time, ultimately leading to the development of an acute respiratory distress syndrome (ARDS), a common but very severe complication in respiratory tract infections (such as COVID-19).