Scientific Calendar February 2025
Diagnosis of Paroxysmal Nocturnal Haemoglobinuria
What is the role of immunophenotyping in the diagnosis of PNH?
It primarily measures the level of haemoglobin in the blood during haemolytic episodes.
It identifies the presence of specific antibodies that target blood cells in PNH patients.
It is used to detect the absence of glycosylphosphatidylinositol (GPI)-anchored proteins on the surface of blood cells.
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific Background
Paroxysmal nocturnal haemoglobinuria
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare and life-threatening clonal disorder of haematopoietic stem cells characterised by chronic intravascular haemolysis caused by the complement cascade, thromboses and cytopenias related to bone marrow failure. It results from a mutational expansion of a clone which inactivates the X-linked gene PIG-A, producing a deficiency of glycosylphosphatidylinositol (GPI)-linked proteins on the surface of red blood cells (RBC), monocytes and granulocytes. These include two complement regulatory proteins on the red blood cell surface, namely, CD55 (decay accelerating factor – DAF) and CD59 (membrane inhibitor of reactive lysis – MIRL). A deficiency in these two proteins thus leads to the destruction of red blood cells in vivo [1].
Diagnosis
Diagnosis of PNH involves a number of laboratory tests:
- Flow cytometry of GPI-linked antigens illustrating negative RBC and WBC populations
- Coombs test positive
- Anaemia with increased reticulocyte count
- Increased lactate dehydrogenase and bilirubin
- Decreased haptoglobin
- Free serum haemoglobin and haemoglobinuria
- Associated organ damage from haemolysis and / or thrombosis [1].
Immunophenotyping by flow cytometry, using the DryFlowEx PNH High-Sensitivity Assay Kit
Flow cytometry is considered the gold standard for the detection of PNH clones, thus playing an important role in the diagnosis, monitoring, and clinical management of patients with PNH. The most recent update of the ICCS/ESCCA consensus guidelines [summarised in 2] ensures sensitive and accurate detection of even minor PNH clones.
The DryFlowEx PNH High-Sensitivity Assay Kit has been developed following this most recent update of the ICCS/ESCCA consensus guidelines, which allows for a high level of detection sensitivity, in part, due to the inclusion of FLAER (Alexa Fluor™ 488-labelled proaerolysin). FLAER binds to GPI anchors, and thus allows measurement of the presence or absence of these, independently of a particular CD marker, on monocytes and neutrophil granulocytes.
The kit provides two tubes per patient:
- RBC tube, containing CD71, CD59 and CD235a
- WBC tube, containing Proaerolysin, CD157, CD45, CD64, CD24, CD14 and CD15
The cocktails for each tube are dry reagents, meaning the antibodies are dried onto the tube surface. This allows for an extended shelf life and room temperature storage and delivery. Details about sample preparation, gating and interpretation are covered in the IFU.
Case and results
A 19-year-old African-American woman presented to an emergency department with a cough and dark urine, she was otherwise previously healthy. Family history showed that her sister had been previously diagnosed with sickle cell trait.
Haematology results
A blood sample was sent to the haematology laboratory where a complete blood count was performed on a XN-Series analyser. The results (Fig 1) showed the patient had an anaemia.
| Rerun | Order | Run | Test | Result | C | Unit |
| I | 2 | CBC Profile | ||||
| I | 2 | HGB | 90 | g/L | ||
| I | 2 | WBC | 2.56 | 10*9/L | ||
| I | 2 | PLT | 69 | & | 10*9/L | |
| I | 2 | RBC | 2.48 | 10*1… | ||
| I | 2 | HCT | 0.277 | L/L | ||
| I | 2 | MCV | 111.7 | fL | ||
| I | 2 | MCH | 36.3 | pg | ||
| I | 2 | MCHC | 325 | g/L | ||
| I | 2 | RDW-CV | 19.9 | % | ||
| RET Profile | ||||||
| R | 2 | RET# | 70.7 | 10*9/L | ||
| R | 2 | RET% | 2.9 | % | ||
| R | 2 | RET-He | 28.3 | pg | ||
| A | 2 | PLT-O | 69 | 10*3/… |
Fig. 1 Haematology lab results from an XN-Series analyser
In summary, the parameters relevant for this case, with hospital-internal reference intervals shown (in parentheses), were:
| 90 g/L | (115 – 155 g/L) |
| 2.48 x 1012/L | (3.9 – 5.6 x 1012/L) |
| 0.277 L/L | (0.36 – 0.48 L/L) |
| 2.9% | (0.5 – 1.5%) |
Results of other tests
Other laboratory tests confirmed the anaemia was autoimmune haemolytic in nature:
| 3170 U/L | (87 – 225 U/L) |
| 1.9 mg/dL | (0 – 1.2 mg/dL) |
| 1.6 mg/dL | (0.2 – 0.7 mg/dL) |
|
Flow cytometric immunophenotyping results
The patient was seen by a Haematologist who sent samples for PNH testing to the flow cytometry laboratory. Immunophenotyping using the DryFlowEx PNH High-Sensitivity Assay Kit was performed. Analysis of the data showed a significant RBC and WBC PNH clone, clearly showing the absence of the GPI-linked antigens.
The results for red blood cells, using the RBC tube, revealed a 76.75% Type III clone and 2.54% Type II clone (Fig. 2).

Results for white blood cells, using the WBC tube, showed a neutrophil granulocyte-clone between 95 and 97% (Fig 3) and a monocyte clone of 98% (Fig 4).
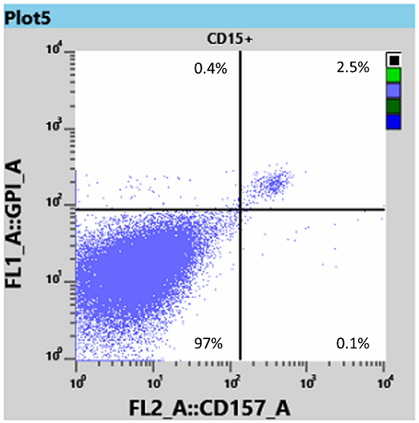
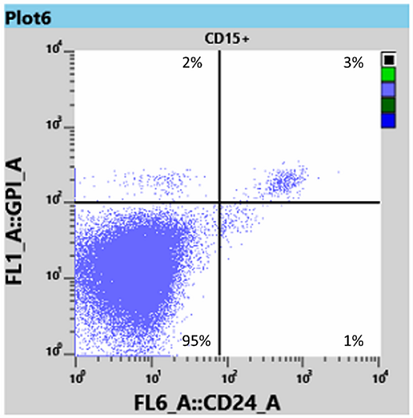
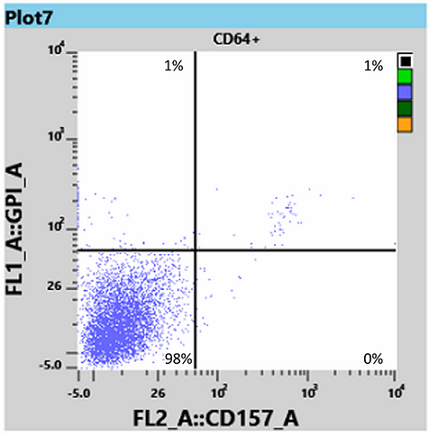
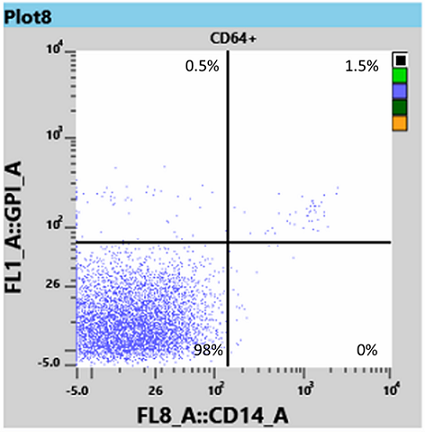
From this information the Haematologist was able to determine that the patient had both homozygous (Type III) and heterozygous (Type II) forms of PNH, and placed the patient on Eculizumab, an immune therapy which binds to the complement C5 protein and inhibits activation of the complement system [3]. This binding prevents the breakdown of red blood cells in the bloodstream.
During the last six months the patient’s haemoglobin and red blood cell count have stabilised into the normal range and there have been no further haemolytic episodes.
References
[1] Cançado RD et al. (2021): Consensus statement for diagnosis and treatment of paroxysmal nocturnal haemoglobinuria. Hematol Transfus Cell Ther; 43(3):341-348. doi: 10.1016/j.htct.2020.06.006.
[2] Illingworth AJ, Marinov I, Sutherland DR (2019): Sensitive and accurate identification of PNH clones based on ICCS/ESCCA PNH Consensus Guidelines-A summary. Int J Lab Hematol; 41 Suppl 1:73–81. doi: 10.1111/ijlh.13011. PMID: 31069981.
[3] McKeage K (2011): Eculizumab. Drugs; 71:2327–2345. https://doi.org/10.2165/11208300-000000000-00000