Sysmex integrative solutions for prostate cancers
In Europe, more than 600,000 men are diagnosed with a urological malignancy each year, with prostate cancer (PCa) being the most frequent [1]. If found early, urological cancers are highly treatable and, in some cases, surgery alone can be curative [2-3]. Our solutions are designed to help the laboratories, clinicians, and patients that address screening, diagnosis and treatment of male cancers.
Read the inspiring story of Ronnie, a prostate cancer survivor
He shares words of inspiration, hope and how this disease changed his life.
Genetic testing for inherited prostate cancer
There is an increasing implementation of genetic counselling and testing in early detection and PCa management [4]. Genetic somatic testing is performed on the tumour while germline testing, which identifies inherited mutations, is performed on blood or saliva.
Germline mutations, such as in BRCA1 and BRCA2 genes, can drive the development of aggressive PCa. Therefore, according to the 2021 EAU guidelines [5] germline testing should be considered for:
- Men with metastatic PCa;
- Men with high-risk PCa and a family member diagnosed with PCa at age < 60 years;
- Men with multiple family members diagnosed with csPCa at age < 60 years or a family member who died from PCa cancer;
- Men with a family history of high-risk germline mutations or a family history of multiple cancers on the same side of the family.
Current research in this field is exploring how genetic testing can support screening, early detection and treatment for mutation carriers and family members.
SureSeq myPanelTM* NGS Custom Prostate Cancer Panel can support in investigating BRCA1, BRCA2 and other relevant genetic mutation using next-generation sequencing.
For further information, visit SureSeq myPanel™ NGS Custom Prostate Cancer Panel website
*For research use only; not for use in diagnostic procedures.
Why go blind if you can use a map?
The standard surgery for prostate cancer often includes extended pelvic lymphadenectomy (ePLND) which may lead to serious post-surgical morbidity. Currently, a less invasive alternative is being evaluated in several trials, which uses a sentinel lymph node (SLN) biopsy approach, with very promising outcomes [6-8].
Indeed, there are alternatives to the radioactive method − current standard used in most tracer-guided lymph node mapping approaches. Sentimag® - Magtrace® system is one of them that detects the sentinel lymph nodes magnetically. The Magtrace® liquid tracer is injected into the prostate pre-operatively. During surgery, the detection of sentinel lymph nodes potentially harbouring metastasis is performed with the help of the magnetometer Sentimag®, which can be used for both in vivo identification and ex vivo confirmation of magnetic lymph nodes. Using this approach, one pilot study involving 19 patients showed a median detection of 7 SLNs, whereas the median of excised lymph nodes from ePLND was 17 [9]. A follow-up study involving 50 patients was conducted, showing a detection rate of 100% and a median of 9 excised SLNs [10]. The Sentimag probe is available for open surgery only.
A pre-operative mapping via visualisation of the lymph nodes can further facilitate the identification – even beyond the patterned areas often used to perform ePLND. After transrectal injection of Magtrace® into the prostate, a pre-operative MRI maps out the sentinel nodes due to the uptake of superparamagnetic iron oxide particles (SPIO) and can be used as further guidance to the nodes of interest during surgery [11].
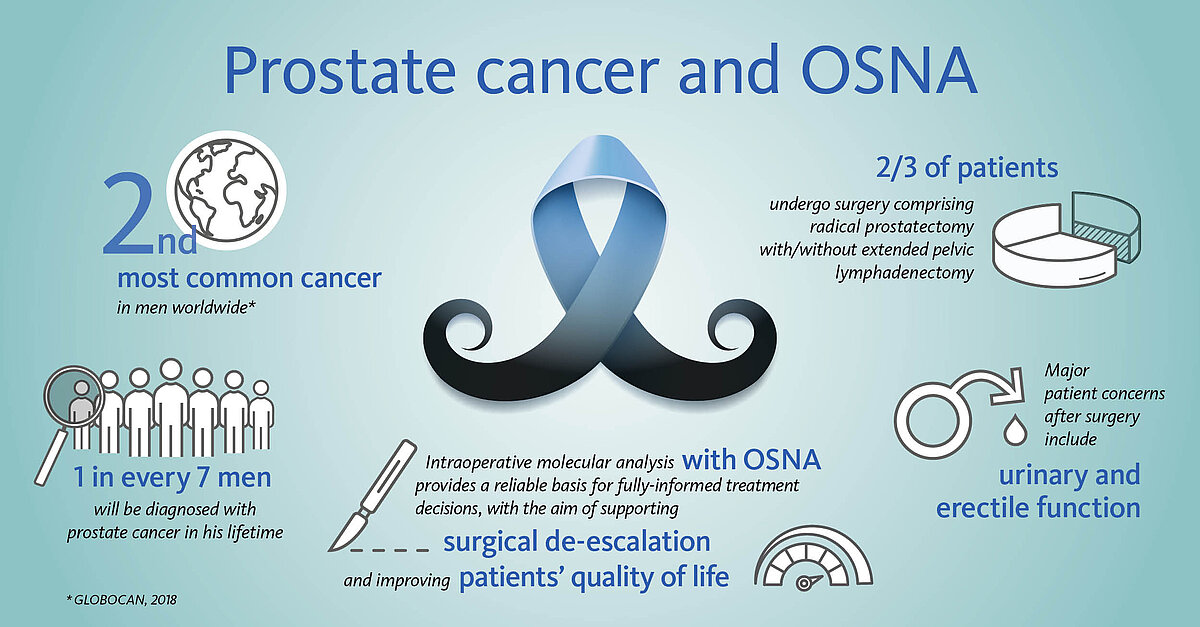
Accurate intraoperative lymph node analysis with OSNA supports de-escalation of surgical radicality
Most men undergoing surgery will have not only their prostate removed, but also the pelvic lymph nodes (LN) in order to eliminate any potential source of tumour cells. While the removal of the prostate is associated with risks of urinary and erectile dysfunctions, the removal of the adjacent LNs increases the chances of lymphatic drainage dysfunctions.
However, in some 70-90% of early-stage patients the tumour is confined to the prostate, making the extra burden of the node dissection curatively pointless.
Could extensive LN dissection and related morbidity be spared in these men?
Pioneer groups in the field of prostate cancer advocate innovative, less invasive treatment approaches by reducing the number of LNs that are removed to a bare minimum. Namely the sentinel lymph node biopsy, a well-stablished method in several other malignancies; here, only the node(s) most prone to carry metastasis are removed and analysed [6-8].
The OSNA (one-step nucleic acid amplification) molecular diagnostic test has been successfully applied for the accurate detection of nodal metastases in breast cancer patients worldwide for over 10 years. As of 2020, OSNA technology is also available for prostate cancer patients. OSNA provides fast and accurate results intraoperatively, thus supporting de-escalation of surgery and potentially improving prostate cancer patients’ quality of life.
Revealing the whole picture
Metastatic cancer spread to lymph nodes (LN) is a pivotal prognostic factor in prostate cancer; hence, correct assessment of the nodal status is paramount for adjuvant treatment planning. Current histopathological assessment of dissected nodes comprises H&E staining of a few tissue sections often leaving small metastases undetected, thus impairing staging accuracy. Moreover, despite being more sensitive, histopathological serial sectioning and immunochemistry are known to be labour-intensive and time-consuming, therefore not suitable for intraoperative use.
To overcome such limitations, OSNA constitutes an optimal solution. It is a rapid, easy-to-use, highly sensitive and standardised molecular diagnostic which allows quantification of total tumour load in the entire LNs, especially for low-volume disease. Using OSNA for intraoperative diagnostics can support PCa personalised lymph node surgery. Additionally, OSNA improves nodal staging in PCa while reducing pathologists’ workload, as most processes are automated and can be performed with minimal effort by a skilled technician [12].
On the way to precision therapy
Many genes can become mutated during the onset and development of prostate cancer [1]. Patients identified with certain genetic mutations can benefit from increased monitoring and/or targeted therapy.
Nowadays, a range of anti-tumour drugs targeting specific genetic mutations are available, and can be adjuvant to standard chemo/immuno/hormonal and radiation therapies. To investigate such mutations, oncologists or urologists may request a genetic test by next generation sequencing (NGS) which may support them in making personalised treatment decisions.
SureSeq* myPanelTM NGS Prostate Cancer panel offers customised NGS tests that interrogate critical and currently relevant prostate cancer genes including: ATM, BRCA1, BRCA2 and PALB2. Combined with OGT’s complimentary Interpret NGS analysis software, it ensures an effortless translation of NGS data into meaningful results.
For further information go to: SureSeq myPanel™ NGS Custom Prostate Cancer Panel website
Other critical and currently relevant prostate cancer genetic mutations including TMPRSS2-ERG fusion, SRD (CHD5) deletion and ZNF217 amplification can be interrogated with CytoCell FISH (Fluorescent In Situ Hybridisation) probes, helping oncologists to identify important biomarkers for PCa subtyping and prognostication [13].
For further information go to: CytoCell website
* CytoCell® FISH probes: For laboratory professional use only. Not intended for use as stand-alone diagnostics or companion diagnostics. Therapeutic action should not be initiated on the basis of the FISH result alone.
* SureSeqTM myPanelTM: For Research Use Only. Not for use in diagnostic procedures.
Manufacturer and Trademarks: CytoCell® FISH Probes (Cytocell Limited), SureSeqTM myPanelTM (Oxford Gene Technology IP Limited)
Resources
[1] Ferlay J et al. (2013): Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 49(6):1374-403.
[2] Bekelman JE et al. (2018): Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol.:JCO1800606.
[3] Mottet N et al. (2017): EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 71(4):618-629.
[4] Giri, V.N., et al. (2020): Implementation of Germline Testing for Prostate Cancer: Philadelphia Prostate Cancer Consensus Conference 2019. Clin Oncol, 2020. 38: 2798.
[5] EAU-EANM-ESTRO_ESUR_ISUP_SIOG- (2021): Guidelines-on-Prostate-Cancer
[6] Van der Poel HG et al. (2017): Sentinel Node Prostate Cancer Consensus Panel Group members. Sentinel node biopsy for prostate cancer: report from a consensus panel meeting. BJU Int. 120(2):204–211.
[7] Van der Poel HG et al. (2017): Sentinel node biopsy and lymphatic mapping in penile and prostate cancer. Urologe A. 56(1):13–17.
[8] Wit EMK et al. (2017): Eur Urol. 71(4):596–605. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy.
[9] Winter A et al. (2014): A novel method for intraoperative sentinel lymph node detection in prostate cancer patients using superparamagnetic iron oxide particles and a handheld magnetometer: The initial clinical experience. Ann Surg Oncol. 21:4390–4396.
[10] Winter A et al. (2016): Magnetometer guided sentinel lymphadenectomy after intraprostatic injection of super-paramagnetic iron oxide nanoparticles in intermediate and high-risk prostate cancer patients. J Urol. 195 (Suppl. 4):e987.
[11] Winter A et al. (2016): Magnetic resonance sentinel lymph node imaging in prostate cancer using intraprostatic injection of superparamagnetic iron oxide nanoparticles: The first in-human results. Eur Urol Suppl. 15 (Suppl. 3):1060.
[12] Engels S (2021): Evaluation of Fast Molecular Detection of Lymph Node Metastases in Prostate Cancer Patients Using One-Step Nucleic Acid Amplification (OSNA). Cancers. 13(5):1117.
[13] Arora K, Barbieri CE. (2018): Molecular Subtypes of Prostate Cancer. Curr Oncol Rep. 2018 Jun 1;20(8):58. doi: 10.1007/s11912-018-0707-9. PMID: 29858674.